What is galvanizing?
Galvanizing, as the name implies, is to coat the surface of metals, alloys and other materials with a zinc layer. It is a surface treatment technology that has the effect of beauty and rust prevention. Since zinc is a sacrificial coating, it can protect the underlying substrate. Zinc is easily soluble in acid and alkali, so it is called an amphoteric metal, and it hardly changes in dry air. In humid air, a dense basic zinc carbonate film will form on the surface of zinc. In sulfur dioxide, hydrogen sulfide and marine atmospheres, zinc has poor corrosion resistance, especially in high temperature and high humidity atmospheres containing organic acids, the zinc coating is very susceptible to corrosion. The zinc coating can significantly improve its protective and decorative properties after passivation, dyeing or coating with a light protectant. Therefore, galvanizing is often used to extend the service life of metal parts. This metal coating has a wide range of uses and can be used for parts in different industries such as automobiles, solar energy, electronics and construction.

What types of metals can be galvanized?
The most common metals suitable for galvanizing are steel and iron. However, other types of metals can also be galvanized. Generally speaking, ferrous metals such as cast steel, cast iron, malleable cast iron, hot-rolled steel, and cold-rolled steel can be galvanized.
On the other hand, there are other materials that cannot form a galvanized layer. Since the iron element is important in the galvanizing reaction, it is difficult for metals such as copper or aluminum to form a galvanized layer. In addition, although materials such as chrome-molybdenum steel may form a coating, there will be many exposed areas and the galvanized layer will be incomplete.
Galvanizing process: How does the galvanized layer protect the base metal?
Galvanizing is the addition of a layer of zinc to the surface of metals such as steel and iron. There are many ways to galvanize. Therefore, the process varies depending on the selected technology. However, they all have a common principle, which is to apply zinc around the steel or iron, which can be liquid or powdered.
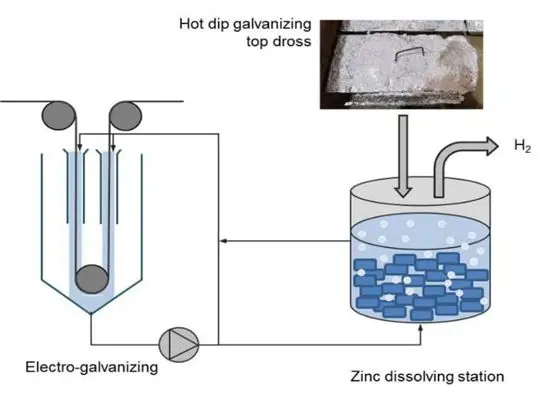
Different Galvanizing Methods
There are a variety of methods for galvanizing, each with its own characteristics and advantages. Let's take a look at them below.
1. Hot-dip galvanizing
As the name suggests, this method involves dipping the base metal into a pool of molten zinc. Before the actual galvanizing, the machinist must chemically or mechanically clean the base metal. Cleaning is a preparatory process to ensure the quality of the bond between the metal and the zinc coating. After cleaning, the metal is fluxed to remove any residual oxides.

Then it is dipped in a heated zinc bath, which is usually kept at around 460°C. When the metallurgical bond between the zinc and the metal base begins to form, after the metal base is fished out of the molten pool, it reacts with oxygen to form zinc oxide. Zinc oxide also reacts with carbon dioxide to form zinc carbonate, which is the final protective layer.
Hot-dip galvanizing is a fast, economical method that can be performed on both simple and complex sheet metal parts. However, if it is not done properly, you may find that the coating on the metal surface is somewhat inconsistent.
2. Pre-galvanizing
The pre-galvanizing process is similar to hot-dip galvanizing. However, it is carried out at the first stage of production (the steel mill). The process prepares the metal for galvanizing by rolling it through a mechanical or chemical cleaning agent. After cleaning, the machinist passes the base metal through a bath of molten zinc and then immediately re-rolls it.
This process ensures that large coils of steel are galvanized quickly, forming a more uniform coating. However, when the pre-galvanized metal is initially manufactured, some uncoated and exposed areas may appear. For example, when a long coil of steel is cut into small pieces, the cut edges are exposed because there is no zinc coating.
3. Cold galvanizing
Cold galvanizing, also known as electrogalvanizing, is different from the two processes discussed above in that it does not require the use of a molten zinc bath. Instead, electroplating involves introducing an electric current into an electrolyte solution before applying it to the substrate. The current acts to transfer zinc ions to the metal substrate. This process creates a precise, uniform coating thickness on the metal. Moreover, the coating of this method is generally thinner than that of hot-dip galvanizing.

There are many different types of electrogalvanizing. But from the pH value of the zinc plating solution, there are mainly two categories: alkaline zinc plating and acid zinc plating.
3.1 Alkaline zinc plating
Alkaline zinc plating process refers to the pH value of the plating solution is alkaline. But due to different complexing agents, it is divided into cyanide zinc plating and zincate zinc plating. Cyanide zinc plating is a very old type of plating. There are three main components in the plating solution: main salt zinc oxide, complexing agent sodium cyanide and conductive salt sodium hydroxide (commonly known as caustic soda). There was no brightener in the early cyanide plating solution. With the improvement of people's aesthetic requirements, brighteners for brightening are added to the cyanide zinc plating solution. The cyanide zinc plating process is stable and the coating is fine. The plating solution has good dispersion ability. According to the different content of sodium cyanide, it is divided into high cyanide, medium cyanide and low cyanide zinc plating. The biggest disadvantage of cyanide zinc plating is that it is too toxic and has serious environmental damage.
Zincate zinc plating is a zinc plating process that has developed rapidly in the past 30 years. Its main components are zinc oxide as the main salt and sodium hydroxide (commonly known as caustic soda), which is a complexing agent and conductive salt. In order to obtain a bright coating with fine dispersion ability, a brightener must be added. The main development period of zincate zinc plating in China was the cyanide-free zinc plating era in the 1970s. The famous DPE zinc plating process and DE zinc plating process have been used until now. Although this process is not as stable and delicate as the cyanide zinc plating process, its biggest advantage is that it is cyanide-free. The harm to the environment is much smaller. Now zincate zinc plating has made new developments, and defects such as blistering and brittleness have been overcome, and the dispersion ability has been greatly improved, which can be compared with cyanide zinc plating.

3.2 Acidic zinc plating
The pH value of acidic zinc plating solution is 4-6. The second result of the cyanide-free zinc plating in the 1970s was cyanide-free ammonium chloride zinc plating. It is on par with zincate alkaline zinc plating. It uses zinc chloride as the main salt, ammonium chloride as the complexing agent and conductive salt. Citric acid and nitrilotriacetic acid are auxiliary complexing agents. Polyethylene glycol and thiourea are used as coating refiners. The coating obtained from this plating solution is fine and has good dispersibility. The plating solution is less toxic. However, the main disadvantage is that the plating solution is unstable, and the ammonium chloride gas emitted is very corrosive to the electroplating equipment. The current efficiency is also low. It is highly sensitive to impurities. The operating temperature range is narrow. It has now been basically eliminated by the potassium chloride zinc plating process.
There is also a sulfate zinc plating process in the acid zinc plating process. Its main component is the main salt zinc sulfate. The content is between 250-500g/L. Alum or aluminum sulfate is used as a conductive salt. The content is between 30-50g/L. Sodium sulfate or sodium chloride is also added to the conductive salt. In addition to the above, some additives are also added to make the coating fine. Dextrin or gum was used at first, and later some special brighteners were invented to make the coating brighter and finer. The biggest advantage of sulfate zinc plating is that it can use a larger current density (1-5A/dm2) and a fast plating speed. But the disadvantage is that the dispersibility of the plating solution is very poor, and it is not suitable for plating more complex workpieces. Due to its particularity, sulfate galvanizing is mainly used in industries with simple shapes and strong continuous production, such as iron wire, steel strip, and steel plate.
4. Annealing galvanizing
Galvanizing annealing is a combination of hot-dip galvanizing and annealing processes. The purpose of this process is to coat the galvanized steel with a special coating. The annealing and hot-dip processes are carried out in an instant to produce a matte gray surface.
The hot-dip process is usually carried out before the plated metal passes through an air knife, which helps to remove excess zinc from the metal. The metal is then briefly heated at a temperature of 500 to 565 ° C in an annealing furnace, and the zinc and iron layers diffuse into each other to form a zinc-iron alloy on the surface.
The galvanized steel sheets produced by this method can withstand welding well, and their surfaces can also ensure excellent paint adhesion.
Galvanizing process
When zinc is introduced to the component, the iron in the metal substrate reacts with the zinc to form a tightly bonded alloy coating. This is a relatively simple coating that forms a fairly thick coating. The entire process can be divided into three stages:
1. Preparation of the metal surface
Galvanizing process
Surface treatment after galvanizing

2. Generally speaking, galvanizing protects metals based on the following principles
The zinc layer can protect the base metal from corrosive substances such as acids, caustics, and gases.
Zinc is a sacrificial metal. When the coating is scratched, zinc easily sacrifices its anode, thereby protecting the base metal from rust.
Zinc corrodes faster than the base metal and is an excellent way to protect metals.
However, the preliminary preparation work and post-treatment methods will determine the effect of the galvanizing process. Inadequate preparation will affect the reaction between the base metal and the molten steel. In addition, inadequate post-treatment will also affect the final appearance of the galvanized layer. Thus affecting the overall effect of the product.
Reference
https://www.cnzxhj.com/a/138259.html
https://finance.sina.cn/futuremarket/qsyw/2019-01-11/detail-ihqhqcis5265360.d.html
https://waykenrm.cn/blogs/what-is-galvanizing/
https://www.163.com/dy/article/F5BMFBON053875K1.html